

BLOGS > FEBRUARY 13, 2024
BY CHRISTOPHER TALBOT
The first topic in the new IB Biology syllabus is A1.1 Water. Water featured as a subtopic in the previous syllabus but is now present in its own expanded topic that explores water at a deeper level with more explicit links to other parts of the syllabus, especially the origin of life.
Students will enter IB Biology with varying levels of understanding of water and its properties, depending on their previous studies. An understanding of some of the biological properties of water depends on some knowledge of chemistry and physics.
This blog shows how inquiry-based approaches can be used to teach this topic, using a series of activities. Some common misconceptions are also outlined, as well links to ToK.
A1.1.2 Hydrogen bonds as a consequence of the polar covalent bonds within water molecules
Activity 1
An introductory question as a starting activity could be for students to: Write down and summarise what they already know about the behaviour and properties of water. (These all of course depend on the presence of relatively strong hydrogen bonds).
This could be followed up with a ”circus” of practical activities, such as:
Students could be asked to deduce conclusions about the properties observed.
Activity 2
This is a simple activity where students put ice on liquid water.
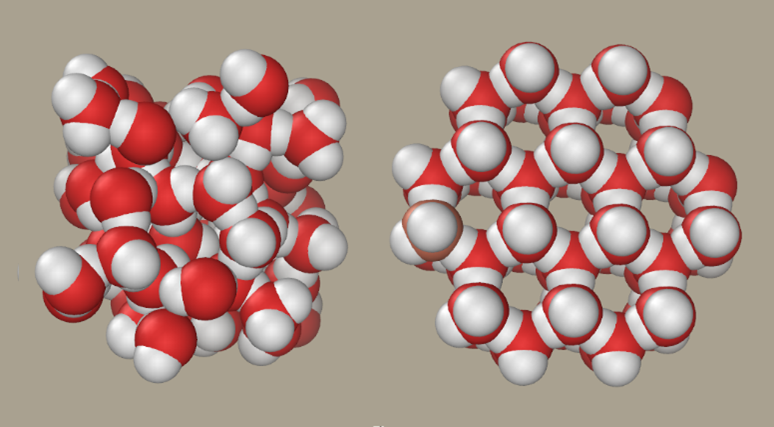
Figure 1 The structures of water and ice (https://commons.wikimedia.org/wiki/File:Liquid-water-and-ice.png)
A student discussion could be: Imagine that ice did not float on water. Predict how you think that would affect the biological world?
The unique properties of water and its ability to form crystalline ice with a regular and ordered lattice is believed to depend on its almost tetrahedral distribution (Figure 2) of electron pairs (two bonding pairs) and two lone (non-bonded) pairs which allow water to form hydrogen bonds with four other water molecules.
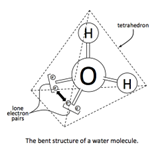
Figure 2 The tetrahedral distribution of electrons pairs around the central oxygen atom of a water molecule (https://commons.wikimedia.org/wiki/File:Tetrahedral_Structure_of_Water.png)
An extension of the practical activity is for students to investigate the relative densities by adding ice to ethanol and then ice to water. Students will enjoy varying the proportions of water and ethanol until the ice ‘floats’ in the middle of the liquid in its container. Unfortunately, a freezer (around -20 °C) is not cold enough to freeze ethanol (freezing point of -114 °C).
Activity 3
A Molymod kit could be used to model the structure and the V-shape of water molecules (Figure 3). This could be used to introduce or remind students about chemical concepts such as atom, electron, covalent bond (sharing of electron pairs), lone pair (non-bonded pair) and molecule.
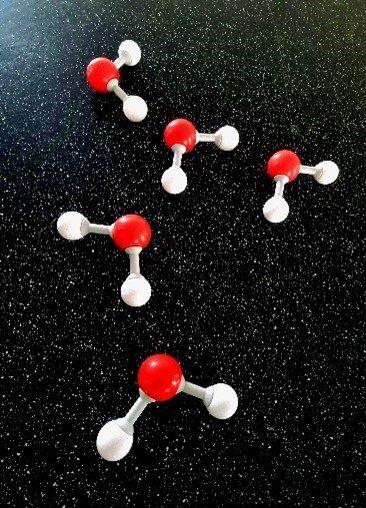
Figure 3 Molymod kit showing water molecules
Activity 4
Deflection of a polar liquid (following a risk assessment), e.g., propanone, ethanol, and water can be achieved by running these liquids through separate burettes and bringing a freshly rubbed dry polythene rod close to the stream as it falls into a beaker below. The experiment works best when there is low humidity and if the polythene rods have been thoroughly dried with a hairdryer prior to use. There must be no naked flames in the laboratory.
Activity 5
One possible student activity is for students to research and compare and contrast the physical properties of water (H2O) and hydrogen sulfide (H2S), which have similar molecular structures. The crucial difference is that hydrogen sulfide does not engage in hydrogen bonding, but its molecules are associated by a similar but weaker interaction known as a dipole-dipole force.
The importance of hydrogen bonding in nucleic acids and protein folding should also be mentioned to “sign post” future topics and concepts, such as base pairing in nucleotides, alpha helices and the beta pleated sheets in proteins.
Activity 6
Students will be very familiar with the solvent properties of water which can best be explained by using a commercial water kit (Figure 4) involving molecular models to explain the solubility of sodium and chloride ions (via hydration of ions) and the low solubility of ethane (due to its non-polar nature and its inability to hydrogen bond with water molecules).

Figure 4 Water model kit
This hydration process (Figure 5) occurs because the partially negatively charged oxygen atoms of water molecules are attracted to the positive sodium ions; conversely, the partially positively charged hydrogen atoms of water molecules are attracted to the negative chloride ions.
The forces of attraction between the water molecules and the ions are stronger than the forces of attraction between the ions. The hydration shell of water molecules also effectively screens the interionic electrostatic attraction and prevents precipitation of the salt.

Figure 5 The modeling of hydrated ions by water molecules (the chloride ion is green and the sodium ion is dark blue)
Activity 7
The role of water as a reactant and product in hydrolysis and condensation reactions could be introduced, using Molymod kits or at a simpler level using interconnecting bricks.
Activity 8
The thermal properties of water can be demonstrated to students using simple experiments:
A1.1.3 Cohesion of water molecules due to hydrogen bonding and consequences for organisms
Activity 9
The cohesion of water can be demonstrated by students slowly dropping water onto a coin (or leaf with thick cuticle) and observing the water droplets forming a drop by cohesion. Eventually the drop breaks and spills off the coin under the force of gravity.
Activity 10
Cohesion can also be demonstrated by students carefully filling a glass to the top and then very carefully adding more water until there is an arc of water slightly over the glass. There will come a point when the cohesive forces are overcome and the water spills over.
A1.1.4 Adhesion of water to materials that are polar or charged for organisms
Students should be made clear about the distinction between cohesion and adhesion. Cohesive and adhesive forces are important for the transport of water from the roots to the leaves in plants via the xylem. Water can adhere to other substances via hydrogen bonding, e.g., with cellulose, but adhesion will also involve weaker interactions, known as London (dispersion) forces.
Activity 11
The adhesive properties of water resulting in capillary action can be demonstrated by placing fine capillary tubes into water stained with a dye.
Additional Higher Level
A1.1.7 Relationship between the search for extraterrestrial life and the presence of water
Activity 12
The IB Biology syllabus implies that life can only evolve in the presence of liquid water. On that basis students could be asked to suggest which places in the solar system have or might have liquid water. Alternatively, each small group could be given one of the following to investigate: Mars, Ceres, Europa, Ganymede, Callisto, Enceladus, Titan, Triton or Pluto.
A table could be generated along the following lines:
Misconceptions
Students often have a number of documented misconceptions about hydrogen bonding, which teachers should be aware of, and the correct scientific concept:
Theory of Knowledge (TOK)
A TOK case study for confirmation bias (or pathological science) is polywater. In 1961, the Soviet physicist Nikolai Fedyakin, who had been repeatedly forcing ultra-pure water through very thin quartz tubes, discovered small quantities of a type of water with significantly different properties to normal water, e.g., higher boiling and lower freezing point. Polywater was suggested to be a covalently bonded polymerised form of water.
Students could be encouraged to research this topic and outline how the story ends.
Learn more about Skills-Based Learning in this blog by Andrew Davis.
Resources and Acknowledgements
For more information, visit titlewave.com.
About the Author:

Chris Talbot has taught chemistry, biology and TOK at schools in Singapore for more than 20 years. He is the author of numerous science textbooks, including Biology for the IB Diploma, Third Edition.
An Author Interview with Drew Daywalt
June 27, 2025
Drew Daywalt, award-winning author of the best-selling The Day the Crayons Quit series, is about to release his second middle grade book with illustrator Mike Lowery, No Sam! and the Meow of Deception. The title continues the hilarious adventures of Sam...
Read more
An Author Interview with Adam Wallenta and Makana Wallenta
June 27, 2025
Get ready to rock the galaxy with the first volume of Punk Taco – a wildly imaginative, music-fueled sci-fi adventure from father-son duo Adam and Makana Wallenta. Created when Makana was just five years old, this award-winning graphic novel now debuts...
Read more
An Author Interview with Lisa Manuzak Wiley
June 27, 2025
A bewitching new graphic novel series is arriving this fall!Author-illustrator Lisa Manuzak Wiley, who grew up in Hawaii, blends cozy fantasy, sisterhood, and tropical charm in a heartfelt homage to her roots: The Witches of Pepperwood Bay Vol. 1. Lisa...
Read more
What We're Reading – Books to Add to Your TBR List
June 4, 2025
As a Follett Content Outside Sales Consultant, I’m not only an avid reader, but also a passionate book reviewer! I’ve curated my top 10 book picks that are perfect for adding to your To Be Read (TBR) list. These titles...
Read more
Author Joseph Koszary on the Changes Made to the International Baccalaureate Extended Essay
May 22, 2025
As someone who’s served as an extended essay coordinator, examiner, and supervisor, I’ve grown deeply familiar with the previous incarnation of the extended essay (EE). Like many of you, years of accumulated experience have made supporting students through the process...
Read more
Celebrate Literacy All Year Long: Host an Online Book eFair!
May 12, 2025
Reading and literacy are essential parts of our lives, and there are numerous events throughout the year dedicated to celebrating and promoting these important skills. Hosting a Follett Book eFair is a fantastic way to engage your school community, share the...
Read more